[sdm_download id=”589″ fancy=”0″]
To view the full article click here to login
[TheChamp-Login title=”Login with your Social Account”]
| Published in final edited form as: J Int Neuropsychol Soc. 2009 September ; 15(5): 671–683. doi:10.1017/S1355617709990233. Molecular neurodevelopment: An in vivo31P-1H MRSI study Gerald Goldstein1, Kanagasabai Panchalingam2, Richard J. McClure2, Jeffrey A. Stanley6, Vince D. Calhoun7,8, Godfrey D. Pearlson9, and Jay W. Pettegrew2,3,4,5 1VA Pittsburgh Healthcare System, Pittsburgh, PA 2Department of Psychiatry, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA 3Department of Neurology, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA 4Department of Behavioral and Community Health Sciences, University of Pittsburgh School of Medicine, University of Pittsburgh, Pittsburgh, PA 5Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA 6Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI 7The Mind Research Network, Albuquerque, NM 8Department of Electrical and Computer Engineering, University of New Mexico, Albuquerque, NM 9Department of Psychiatry, Yale University, Hartford, CT Abstract Synaptic development and elimination are normal neurodevelopmental processes which if altered could contribute to various neuropsychiatric disorders. 31P-1H magnetic resonance spectroscopic imaging and structural MRI exams were conducted on 106 healthy children ages 6–18 years in order to identify neuromolecular indices of synaptic development and elimination. Over the age range studied, age-related changes in high-energy phosphate (phosphocreatine), membrane phospholipid metabolism (precursors and breakdown products), and gray matter were found. These neuromolecular and structural indices of synaptic development and elimination are associated with development of several cognitive domains and changes in gray matter volume. Monitoring of these molecular markers is essential for devising treatment strategies for neurodevelopmental disorders. Keywords MRS; Neuroimaging; Metabolism; Cognition; Multiple Regression INTRODUCTION The four major stages that characterize human brain development are: 1) neuronal proliferation; 2) migration of neurons to specific sites throughout the CNS; 3) organization of the neuronal circuitry; and 4) myelination of the neuronal circuitry (Volpe, 1995). |
The third stage of human brain development, organization of the neural circuitry, is most active from the sixth month of gestation to young adulthood. The major events associated with neuronal circuitry organization include: 1) proper alignment, orientation, and layering of cortical neurons; 2) dendritic and axonal differentiation; 3) synaptic development; 4) synaptic elimination (cell death and/or selective elimination of neuronal processes); and 5) glial proliferation and differentiation. These processes overlap with the timing of normal development of cognitive function and the onset of neurodevelopmental and psychiatric disorders such as attention deficit disorder, autism and schizophrenia. Normal synaptic elimination occurs during early adolescence in non-human primates (Rakic et al., 1986; Bourgeois & Rakic, 1993) and humans (Huttenlocher, 1979; Huttenlocher et al., 1982; Huttenlocher, 1990; Huttenlocher & Dabholkar, 1997). Synaptic elimination in non-human primates is generally observed to occur synchronously in all regions (i.e., homochronous) (Rakic et al., 1986) but is heterochronous in humans (Huttenlocher et al., 1997). Normal synaptic elimination is predominantly of presumptive excitatory asymmetric junctions on dendritic spines (Smiley & Goldman-Rakic, 1993) which probably utilize amino acids, such as L-glutamate, as the neurotransmitter (Storm-Mathisen & Otterson, 1990). Perinatal insults, intrauterine disturbances, and perhaps environmental influences in childhood and adolescence can potentially result in disordered neuronal circuitry (Birch & Gussow, 1970). This study focuses on molecular and structural indices related to synaptic development and elimination.
Conclusions
These conclusions are based on cross-sectional analysis and there would be clear advantages to testing our subjects longitudinally. We have done so, and will report the findings in a future publication. We would note though that it is not likely that the cross-sectional data were markedly influenced by cohort effects since all subjects were recruited from the same community over a period of only four years. There is the possibility of gender differences which we could not evaluate here because of small sample sizes. A final consideration is that the study was limited to an axial slice of brain measures of PCr, sPME, sPDE, and NAA and different results may be found at different regional locations. Since we had the capability of obtaining MRS measures from various brain regions, we will present the findings in a future report.
Acknowledgments
This work was supported in part by an NIHCD/NIH HD-39799 grant (JWP). We thank Terry Bradbury for conducting neuropsychological testing. Indebtedness is also expressed to the Medical Research Service and the VISNIV Mental Illness, Research, Education and Clinical Center (MIRECC), Department of Veterans Affairs for support of this work. We thank Harriet Marshman, Deborah Wetzler and Dennis McKeag for help in conducting the study.
REFERENCES
Agranoff, BW.; Hajra, AK. Lipids. In: Siegel, GJ.; Agranoff, BW.; Albers, RW.; Molinoff, PB., editors. Basic Neurochemistry, Molecular, Cellular, and Medical Aspects. Vol. 5th ed. New York: Raven Press; 1994. p. 97-116.
Andreasen NC, Endicott J, Spitzer RL, Winoker G. The reliability and validity of the family history method using family history research diagnostic criteria (FH-RDC). Archives of General Psychiatry 1977;34:1229–1235. [PubMed: 911222]
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Research Bulletin 2008;76:329–343. [PubMed: 18502307]
Arnold DL, Matthews PM, Francis G, Antel J. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: Assessment of the load of disease. Magnetic Resonance in Medicine 1990;14:154–159. [PubMed: 2161982]
Bartha R, Drost DJ, Menon RS, Williamson PC. Comparison of the quantification precision of human short echo time (1)H spectroscopy at 1.5 and 4.0 Tesla. Magnetic Resonance in Medicine 2000;44:185– 192. [PubMed: 10918316]
Birch, HG.; Gussow, JD. Disadvantaged children, health, and nutrition and school failure. New York: Harcourt, Brace & World; 1970.
Birken DL, Oldendorf WH. N-Acetyl-L-aspartic acid: A literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience and Biobehavioral Reviews 1989;13:23–31. [PubMed: 2671831]
Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Sciences 1987;508:333–348. [PubMed: 3326459]
Bourgeois J-P, Rakic P. Changes of synaptic density in the primary visual cortex of the Macaque monkey from fetal to adult stage. Journal of Neuroscience 1993;13:2801–2820. [PubMed: 8331373]
Brockmann K, Pouwels PJ, Christen HJ, Frahm J, Hanefeld F. Localized proton magnetic resonance spectroscopy of cerebral metabolic disturbances in children with neuronal ceroid lipofuscinosis. Neuropediatrics 1996;27:242–248. [PubMed: 8971744]
Buchli R, Martin E, Boesiger P, Rumpel H. Developmental changes of phosphorus metabolite concentrations in the human brain: A 31P magnetic resonance spectroscopy study in vivo. Pediatric Research 1994;35:431–435. [PubMed: 8047379]
Chugani HR, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology 1987;322:487–497. [PubMed: 3501693]
Cleveland WS. Robust locally-weighted regression and smoothing scatterplots. Journal of the American Statistical Association 1979;74:829–836.
Cleveland WS, Grosse E. Regression by local fitting. Journal of Econometrics 1988;37:87–114.
de Beer, R.; van Ormondt, D. Analysis of NMR data using time domain fitting procedures. In: Diehl, P.; Fluck, EG., editors. NMR Basics, Principles and Progress. New York: Springer-Verlag; 1992. p. 201-258.
De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magnetic Resonance in Medicine 1995;34:721–727. [PubMed: 8544693]
deGraaf AA, Bovee WMMJ. Improved quantification of in vivo1H NMR spectra by optimization of signal acquisition and processing and by incorporation of prior knowledge into the spectral fitting. Magnetic Resonance in Medicine 1990;15:305–319. [PubMed: 1975420]
Ebert D, Speck O, Konig A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right
striatum. Psychiatry Research 1997;74:173–176. [PubMed: 9255862]
Eeg-Olofsson O, Kristensson K, Sourander P, Svennerholm L. Tay-Sach’s Disease. A generalized emtabolic disorder. Acta Paediatrica Scandinavia 1966;55:546–562.
Frahm J, Hanefeld F. Localized proton magnetic resonance spectroscopy of cerebral metabolites. Neuropediatrics 1996;27:64–69. [PubMed: 8737820]
Frahm J, Michaelis T, Merboldt KD, Hanicke W, Gyngell ML, Bruhn H. On the N-acetyl methyl resonance in localized 1H NMR spectra of human brain in vivo. Nuclear Magnetic Resonance in Biomedicine 1991;4:201–204.
Frey, KA. Positron emission tomography. In: Siegel, GJ.; Agranoff, BW.; Albers, RW.; Molinoff, PB., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Vol. 5 ed. New York: Raven Press; 1994. p. 935-955.
Geddes JW, Panchalingam K, Keller JN, Pettegrew JW. Elevated phosphocholine and phosphatidyl choline following rat entorhinal cortex lesions. Neurobiology of Aging 1997;18:305–308. [PubMed: 9263196]
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience 1999;2:861–863.
Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF III, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. Journal of Child Psychology & Psychiatry & Allied Disciplines 2007;48:852–862.
Goldstein FB. Biosynthesis of N-acetyl-L-aspartic acid. Journal of Biological Chemistry 1959;234:2702– 2706.
Goldstein FB. The enzymatic synthesis of N-acetyl-L-aspartic acid by subcellular preparations. Journal of Biological Chemistry 1969;244:4257–4260. [PubMed: 5800445]
Hein H, Krieglstein J, Stock R. The effects of increased glucose supply and thiopental anesthesia on energy metabolism of the isolated perfused rat brain. Naunyn Schmiedebergs Archives of Pharmacology 1975;289:399–407.
Hess, H. The rates of respiration of neurons and neuroglia in human cerebrum. In: Kety, SS.; Elkes, J., editors. Regional Neurochemistry. Oxford: Pergamon Press; 1961. p. 200-202.
Hess HH, Bass NH, Thalheimer C, Devarakonda R. Gangliosides and the architecture of human frontal and rat somatosensory isocortex. Journal of Neurochemistry 1976;26:1115–1121. [PubMed: 932717]
Huttenlocher PR. Synaptic density in human frontal cortex. Developmental changes and effects of aging.
Brain Research 1979;163:195–205. [PubMed: 427544]
Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia 1990;28:517–527. [PubMed: 2203993]
Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex.
Journal of Comparative Neurology 1997;387:167–178. [PubMed: 9336221]
Huttenlocher PR, de Courtten C, Garey LJ, Van der Loos LH. Synaptogenesis in human visual cortex-evidence for synapse elimination during normal development. Neuroscience Letters 1982;33:247– 252. [PubMed: 7162689]
Inglese M, Rusinek H, George IC, Babb JS, Grossman RI, Gonen O. Global average gray and white matter N-acetylaspartate concentration in the human brain. Neuroimage 2008;41:270–276. [PubMed: 18400521]
Jansson SE, Harkonen MH, Helve H. Metabolic properties of nerve endings isolated from rat brain. Acta
Physiologica Scandinavica 1979;107:205–212. [PubMed: 539452]
Kadekaro M, Crane AM, Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proceedings of the National Academy of Sciences of the United States of America 1985;82:6010–6013. [PubMed: 3862113]
Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood. Journal
of Clinical Investigation 1957;36:1130–1137. [PubMed: 13449166]
Klunk WE, Xu CJ, Panchalingam K, McClure RJ, Pettegrew JW. Analysis of magnetic resonance spectra by mole percent: Comparison to absolute units. Neurobiology of Aging 1994;15:133–140. [PubMed: 8159259]
Knizley H. The enzymatic synthesis of N-acetyl-L-aspartic acid by a water-insoluble preparation of a cat brain acetone powder. Journal of Biological Chemistry 1967;242:4619–4622. [PubMed: 6061408] Koller KJ, Zaczek R, Coyle J. N-acetyl-aspartyl-glutamate: Regional levels in rat brain and the effects of brain lesions as determined by a new HPLC method. Journal of Neurochemistry 1984;43:1136– 1142. [PubMed: 6470709]
Kreis R. Quantitative localized 1H MR spectroscopy for clinical use. Journal Progress Nuclear Magnetic Resonance 1997;31:155–195.
Lowden JA, Wolfe LS. Studies on brain gangliosides. III Evidence for the location of gangliosides specifically in neurones. Canadian Journal of Biochemistry 1964;42:1587–1594.
McCandless DW, Wiggins RC. Cerebral energy metabolism during the onset and recovery from halothane anesthesia. Neurochemical Research 1981;6:1319–1326. [PubMed: 7339509]
McIlwain, H.; Bachelard, HS. Biochemistry and the Central Nervous System. Vol. 5 ed. Edinburgh: Churchill Livingstone; 1985.
Pettegrew, JW.; Keshavan, MS.; Stanley, JA.; McClure, RJ.; Johnson, CR.; Panchalingam, K. Magnetic resonance spectroscopy in the assessment of phospholipid metabolism in schizophrenia and other psychiatric disorders. In: Peet, M.; Glen, I.; Horrobin, DF., editors. Phospholipid Spectrum Disorder in Psychiatry. Vol. 2nd ed. Carnforth, UK: Marius; 2003. p. 239-255.
Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. Nuclear Magnetic Resonance in Biomedicine 1997;10:73–78.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra.
Magnetic Resonance in Medicine 1993;30:672–679. [PubMed: 8139448]
Rakic P, Bourgeois J-P, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 1986;232:232–235. [PubMed: 3952506]
Rango M, Bozzali M, Prelle A, Scarlato G, Bresolin N. Brain activation in normal subjects and in patients affected by mitochondrial disease without clinical central nervous system involvement: a phosphorus magnetic resonance spectroscopy study. Journal of Cerebral Blood Flow & Metabolism 2001;21:85– 91. [PubMed: 11149672]
Rango M, Castelli A, Scarlato G. Energetics of 3.5 s neural activation in humans: a 31P MR spectroscopy study. Magnetic Resonance in Medicine 1997;38:878–883. [PubMed: 9402187]
Roux L. Magnetic Resonance. Journal of Magnetic Resonance Imaging 1998;8:1022–1023. [PubMed: 9786138]
Seeger U, Klose U, Mader I, Grodd W, Nagele T. Parameterized evaluation of macromolecules and lipids in proton MR spectroscopy of brain diseases. Magnetic Resonance in Medicine 2003;49:19–28. [PubMed: 12509816]
Siesjo, BK. Brain Energy Metabolism. New York: John Wiley & Sons; 1978.
Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 1991;45:37–45. [PubMed: 1754068]
Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: A laminar analysis using the silver enhanced diaminobenzidine-sulfide (SEDS) immunolabeling technique. Cerebral Cortex 1993;3:223–238. [PubMed: 7686795]
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(S1):208–219.
Sokoloff L. Cerebral circulatory and metabolic changes associated with aging. Research Publications Association for Research in Nervous and Mental Disease 1966;41:237–254. [PubMed: 5957649]
Sokoloff L. Measurement of local cerebral glucose utilization and its relation to local functional activity in the brain. Advances In Experimental Medicine & Biology 1991;291:21–42. [PubMed: 1927683] Sokoloff L. Function-related changes in energy metabolism in the nervous system: localization and mechanisms. Keio Journal of Medicine 1993;42:95–103. [PubMed: 8255068]
Stanley JA, Drost DJ, Williamson PC, Thompson RT. The use of a priori knowledge to quantify short echo in vivo1H MR spectra. Magnetic Resonance in Medicine 1995;34:17–24. [PubMed: 7674893] Stanley JA, Pettegrew JW. Post-processing method to segregate and quantify the broad components underlying the phosphodiester spectral region of in vivo 31-P brain spectra. Magnetic Resonance in Medicine 2001;45:390–396. [PubMed: 11241695]
Storm-Mathisen J, Otterson OP. Immunocytochemistry of glutamate at the synaptic level. Journal of
Histochemistry and Cytochemistry 1990;38:1733–1743. [PubMed: 1979340]
Suzuki K. The pattern of mammalian brain gangliosides III. Regional and developmental differences. Journal of Neurochemistry 1966;12:969–979.
Tallan HH, Moore S, Stein WH. N-acetyl-L-aspartic acid in brain. Journal of Biological Chemistry
1956;219:257–264. [PubMed: 13295277]
Truckenmiller ME, Namboodiri MAA, Brownstein MJ, Neale JH. N-Acetylation of L-aspartate in the nervous system: Differential distribution of a specific enzyme. Journal of Neurochemistry
1985;45:1658–1662. [PubMed: 4045470]
Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. Journal of Neuroscience 1993;13:981–989. [PubMed: 8441018]
Whittaker VP. Some properties of synaptic membranes isolated from the central nervous system. Annals of the New York Academy of Sciences 1966;137:982–998. [PubMed: 5229839]
Wiegandt H. The subcellular localization of gangliosides in the brain. Journal of Neurochemistry
1967;14:671–674. [PubMed: 6025100]
Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009;45:S173–S186.
[PubMed: 19059349]
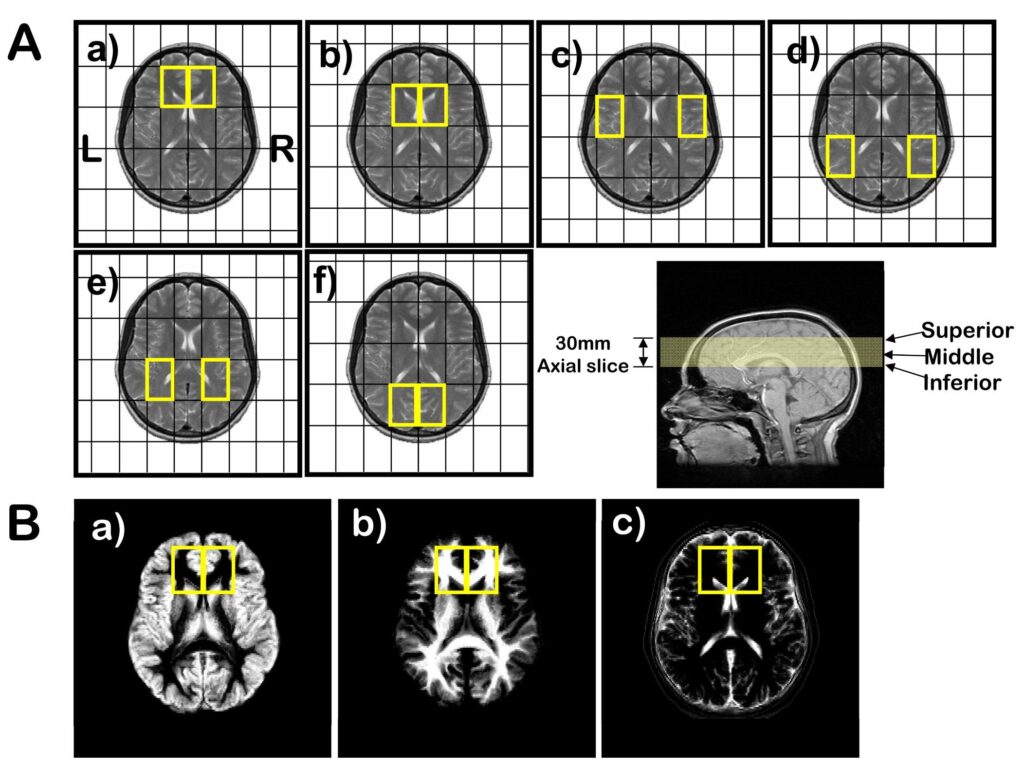
Figure 1.
(A) 31P MRSI voxel grid shifts (outlined in yellow) superimposed on the middle MRI axial slice (bottom right) for: a) prefrontal cortex; b) basil ganglia; c) superior temporal cortex; d) inferior parietal cortex; e) centrum semiovale; and f) occipital regions. Voxel size is 45×45×30 mm3. (B) Segmentation images of: a) gray matter; b) white matter; and c) CSF and extracortical matter where the intensity is proportional to the tissue type of that image. The matrix size of the images is 256 in the sagittal direction by 124 in the coronal direction, reflecting the 124 slices that were acquired for the 3-dimensional SPGR sequence. Right and left 31P prefrontal voxels (yellow boxes) are superimposed on the images.
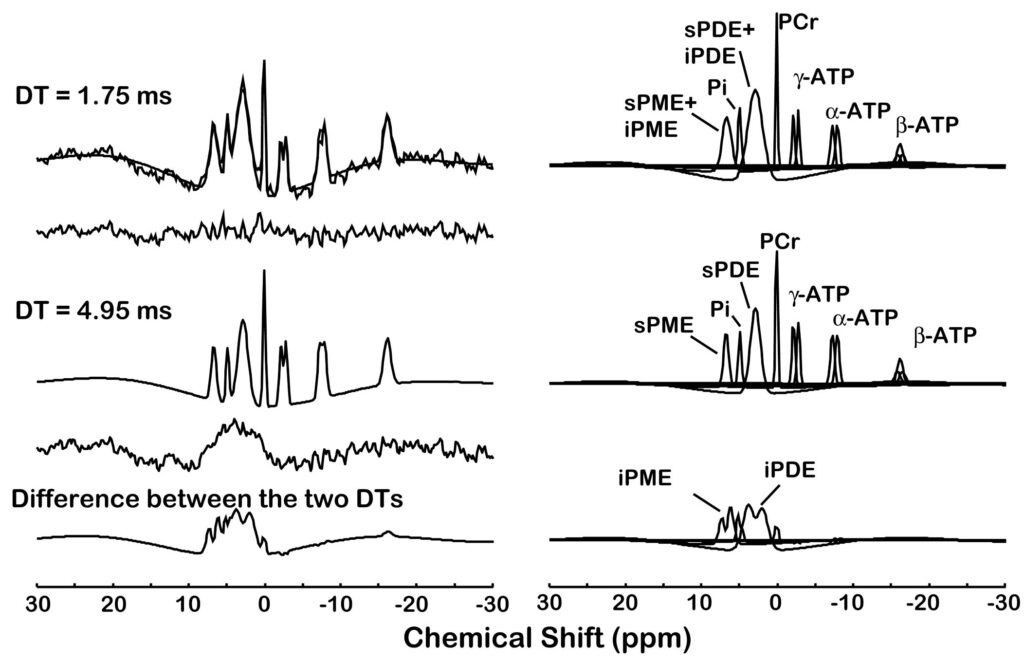
Figure 2.
Quantification of a typical in vivo 31P magnetic resonance spectroscopic imaging spectrum with 5 Hz line broadening from a single voxel (30×30×30 mm3 ) of a study subject. The acquired spectrum is modeled in the time domain with Gaussian-damped sinusoids and by omitting both the first 0.75 ms and first 2.75 ms of the free induction decay using the MarquardtLevenberg algorithm. Both the short (0.75 ms) and long (2.75 ms) delay time (DT) models are shown superimposed on the acquired 31P spectra and the modeled resonances are identified on the right. The difference between the two time domain fits results in the bottom trace containing the intermediate correlation time components.
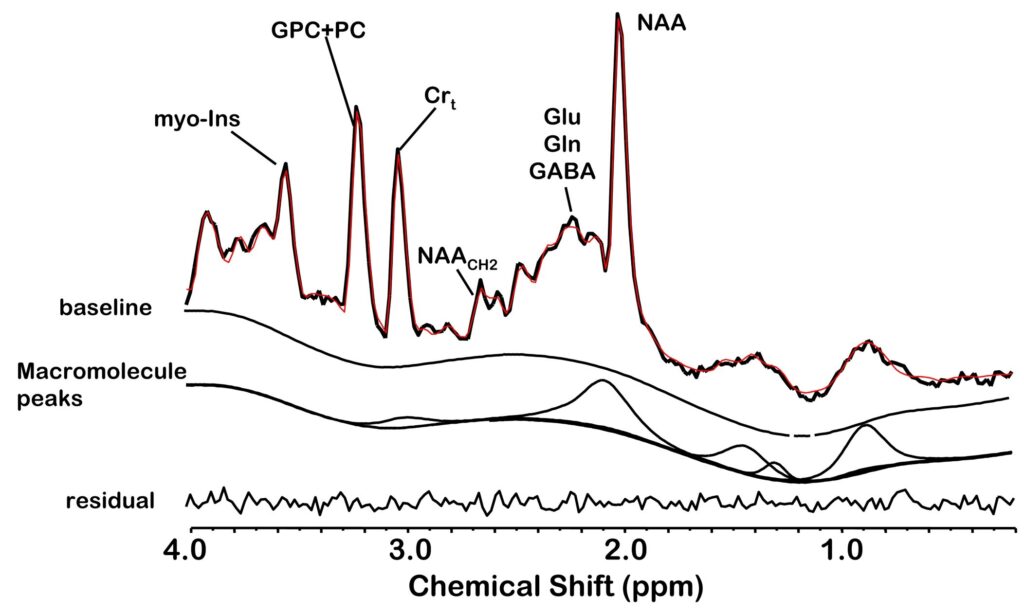
Figure 3.
An example of quantifying a short TE 1H MRSI spectrum of a control subject using the proposed acquisition protocol and LC Model fitting. The acquired spectrum with no line broadening is superimposed on the modeled and baseline spine function and the residual is below. The quantified macromolecule signal is indicated in a separate trace.
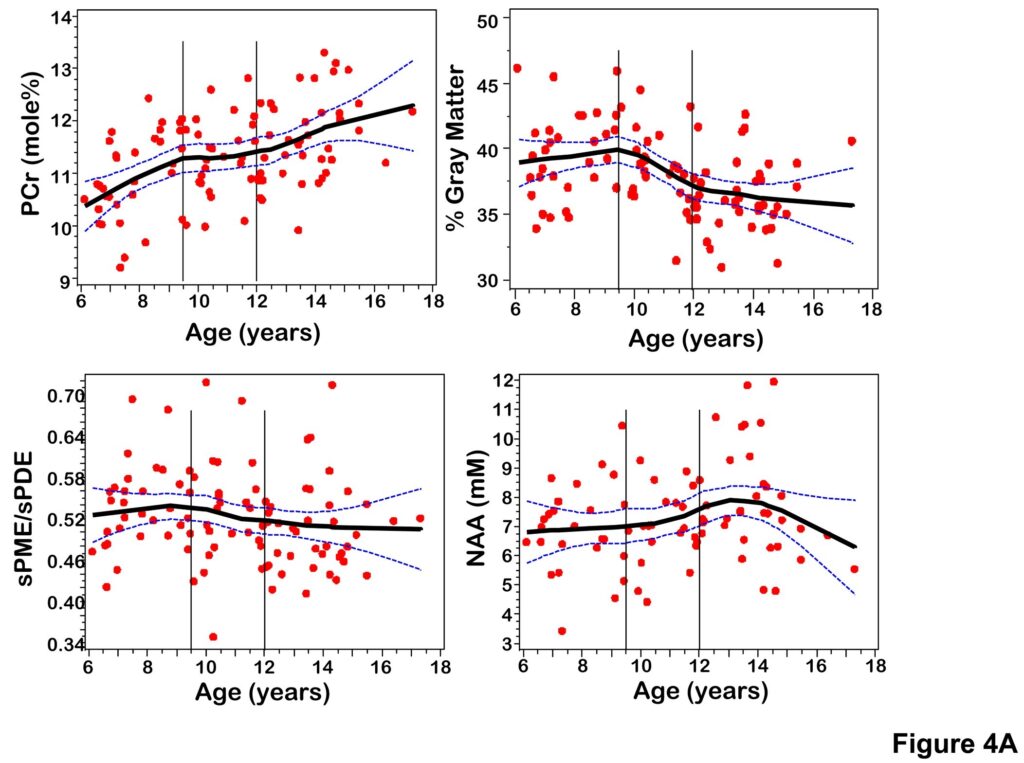
Figure 4.
(A) Scatterplots of PCr, sPME/sPDE, NAA, gray matter volume versus age fitted with a LOESS curve with 95% confidence intervals. (B) Scatterplots of composite scores for cognitive domains (Language, Memory, Visual Spatial, Executive Function) versus age fitted with a LOESS curve with 95% confidence intervals.
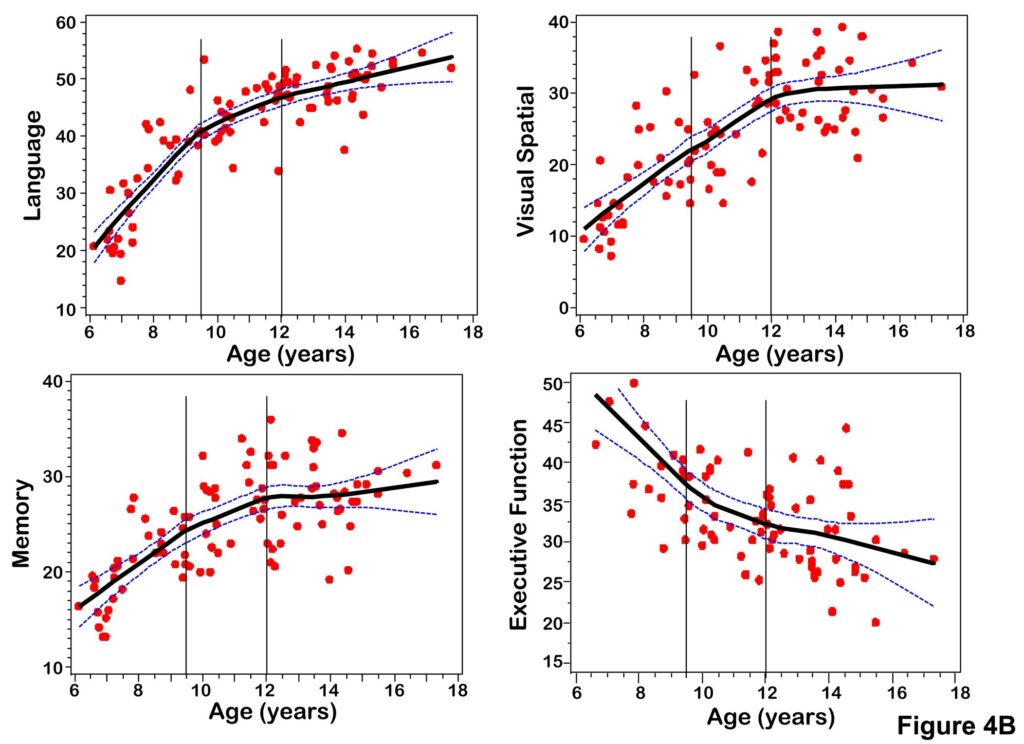
Figure 5.
Z-score plots of PCr, sPME/sPDE, NAA, and gray matter volume versus age.
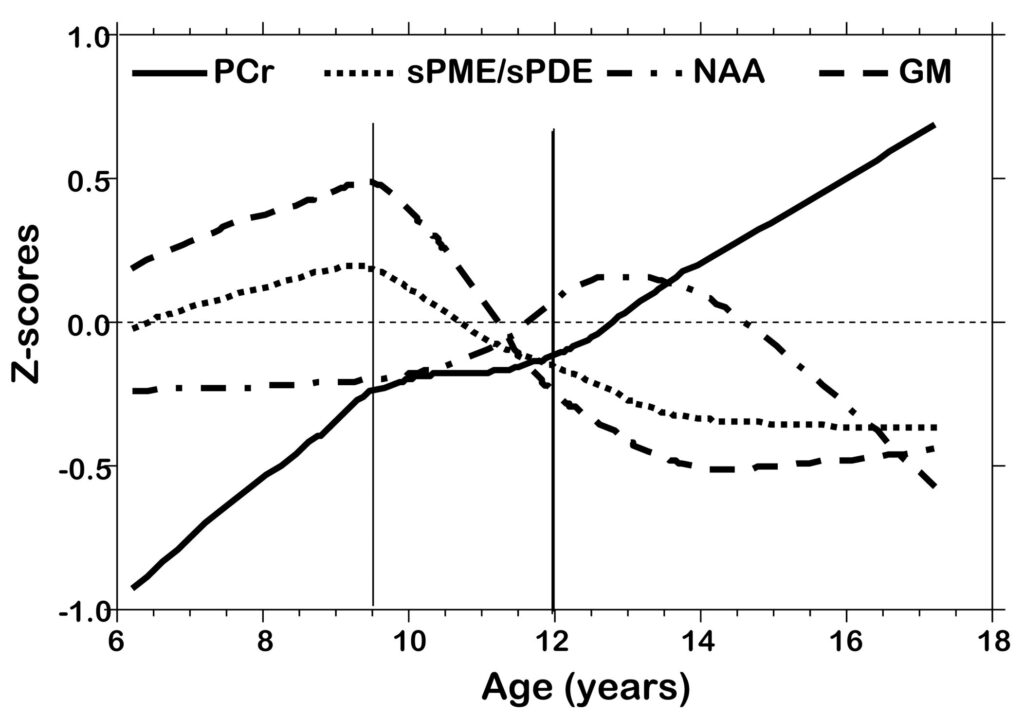
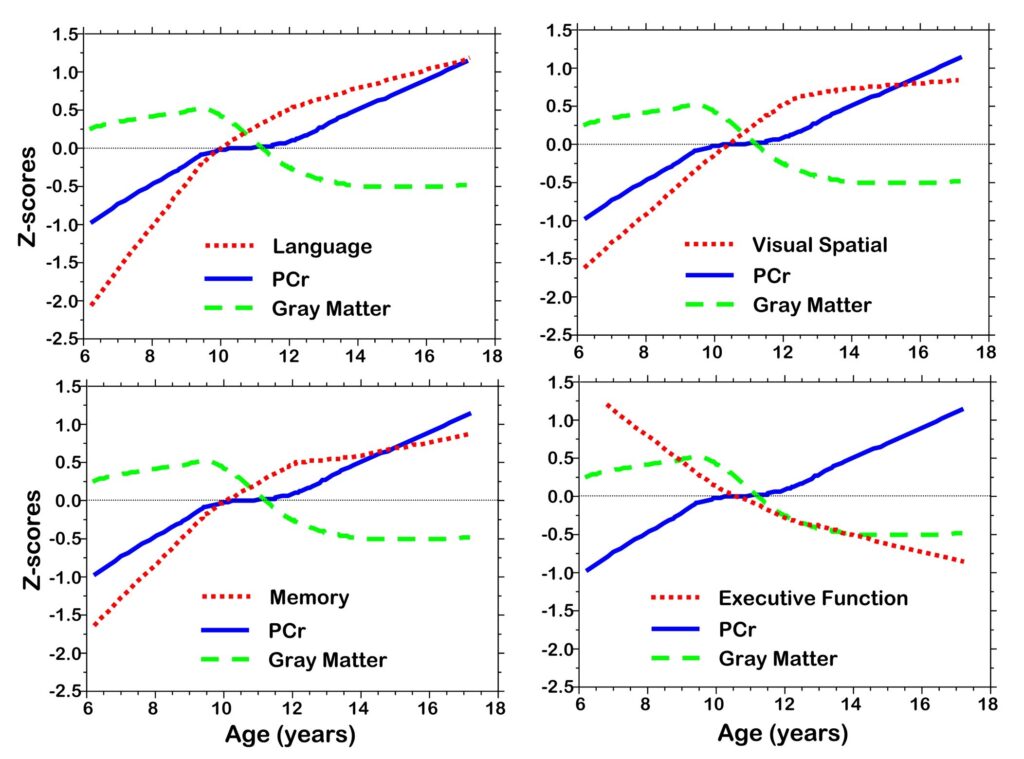
Figure 6.
Z-score plots of PCr, gray matter volume, cognitive domain composite scores (Language, Memory, Visual Spatial, Executive Function).
Table 1
Neuropsychological test variables used within each cognitive domain.
DOMAIN TEST VARIABLES
Language Abbreviated WISC for children Total Verbal raw scores
Wechsler Individual Scale of Intelligence (WASI) for Total Verbal raw scores Adults
Wechsler Individual Achievement Test (WIAT) Reading, Spelling raw scores
Clinical Evaluation of Language Fundamentals (CELF) Concepts and Directions raw scores
Peabody Picture Vocabulary Tests Raw scores
| Executive Function | Wechsler Similarities and Matrix Reasoning Subtests Wisconsin Card Sorting Test | Raw scores Perseverative Errors |
| Visual Spatial | Wechsler Block Design | Raw score |
| Visual Motor Integration Test | Raw score | |
| Test of Visual Perception | Spatial Relations Subtest- raw score | |
| Memory | Wide Range Assessment of Memory and Learning (WRAML) | Picture Memory, Design Memory, Verbal Learning, Story Memory and Number/Letter Subtests |